I.Medical-grade adhesive film reference formulation II. Corresponding explanations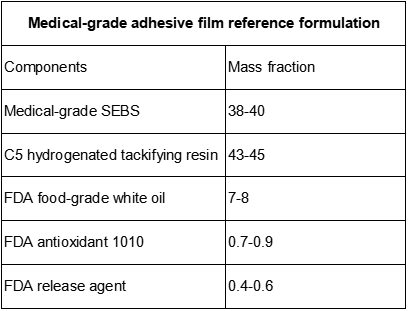
1. Medical-grade adhesive film is mainly used in the inner and outer layers of PP and PE film materials such as infusion bags, blood bags, medical packaging bags, surgical gowns, etc., as a functional adhesive layer, it directly contacts drugs, blood or human tissues, and has extremely high requirements for the safety, cleanliness and compatibility of materials. The reference formulation provided herein, with domestically produced medical-grade SEBS at its core, can significantly reduce production costs while meeting stringent medical standards.
2. The core base of this formula is star-shaped, low-styrene medical-grade SEBS with an addition of 38~40 mass parts. The star-shaped molecular structure gives the film excellent elasticity and molding accuracy, while the low styrene content further improves the biocompatibility of the material and avoids the potential leaching risk caused by high styrene. The use of domestic medical-grade SEBS to replace imported products can not only reduce the cost of raw materials by 20%~30%, but also fully comply with FDA medical contact and food contact standards.
3. The addition of 43~45 mass C5 hydrogenated tackification resin to the formula is the key to achieving high viscosity of the film. C5 hydrogenated resin has the characteristics of high transparency, low odor, and low volatilization, and is highly compatible with medical-grade SEBS, which can greatly improve the adhesion of the film to PP and PE film materials without compromising cleanliness, ensuring that the composite layer does not delayer or fail during long-term use or disinfection.
4. In order to adjust the softness and hardness of the film and the processing fluidity, the formula introduces 7~8 quality FDA food-grade white oil. The white oil is refined by deep hydrogenation process, with a very low aromatic hydrocarbon content (≤0.01%), no fluorescence, no odor, and meets the high cleanliness requirements of medical-grade materials. By accurately controlling the amount of white oil, the film can maintain appropriate flexibility while avoiding problems such as oiliness and stickiness.
5. In order to ensure the stability of the film in processing and long-term use, FDA Antioxidant 1010 with a mass content of 0.7~0.9 and FDA release agent with a content of 0.4~0.6 quality are also added to the formula. Antioxidant 1010 can effectively inhibit the oxidative degradation of materials during high-temperature processing and irradiation disinfection, and prevent the film from turning yellow and brittle. The release agent can improve production efficiency, avoid the film sticking to the mold during extrusion, and do not affect the transparency and adhesive properties of the film.
6. The medical-grade adhesive film prepared by this formula has the characteristics of high cleanliness and high transparency, and all indicators can pass FDA, ISO13485 and other medical certifications, and can be directly used in the composite process of high-end medical products such as infusion bags and blood bags.