Normal crosslinked polymers cannot be recycled, because they don't melt. They don't melt, because the crosslinks tie all the polymer chains together, making it impossible for the material to flow.
This is where the reversible crosslink comes in. Normal crosslinks are covalent, chemically bonding the polymer chains together into one molecule. The reversible crosslink uses noncovalent, or secondary interactions between the polymer chains to bind them together. These interaction include hydrogen bonding and ionic bonding.
The crosslinks using noncovalent interactions are broken when being heated. This allows the material to be processed, and most importantly, recycled. When it cools again, the crosslinks reform. Two approaches have been tried to make a thermoplastic elastomer, ionomers and block copolymers.
Ionomers are a kind of copolymer. They are copolymers in which a small portion of the repeat units have ionic pendant groups attached to them. Normally, the polymer backbone chain will be nonpolar. The “like dissolves like” rule works here, too. The nonpolar polymer backbone chains will group together, and the polar ionic pendant groups will cluster together. Although the cluster of ionic groups would like to separate themselves completely from the nonpolar backbone chains, they can't. They're just sort of attached to the backbone chains. Thus, these clusters of ionic groups serve to tie the backbone chains together, just like a normal crosslink would.
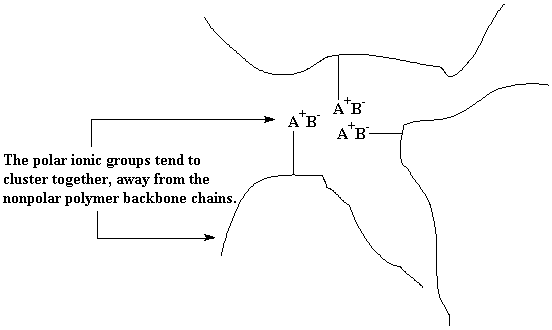
The difference is that the ionic clusters will break up if the ionomers are heated up. When molecules get hot, they move around more, and this motion of molecules is heat itself. Moving around like this at high temperatures makes it hard for the ionic groups to stay in their little clusters, and so they break up. Now the ionomer has lost its crosslinks, and can be processed and recycled just like an ordinary polymer. Cool it back down, and the ionic clusters form again, and it acts like a crosslinked polymer again.
The other way to make a thermoplastic elastomer is called a block copolymer. A copolymer is a polymer made from more than one kind of monomer, i.e. made out of two or more comonomers. A block copolymer is a copolymer in which the comonomers are separated into long sections of the polymer backbone chain. Each of these sections, called blocks, looks sort of like a homopolymer.
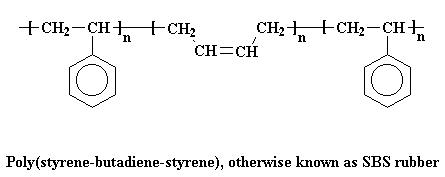
A very common thermoplastic elastomer that is a block copolymer is SBS rubber. SBS stands for styrene-butadiene-styrene, because SBS is made up of a short chain of polystyrene, followed by a long chain of polybutadiene, followed by another short chain of polystyrene. A chain of SBS will look like the picture below after being stretched out.
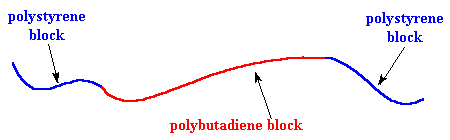
Different polymers don't mix very well as the "like dissolves like" rule says. In fact, it's very difficult to mix two different polymers, even when they are very similar. This holds for the blocks of SBS just as for any other polymers. So the polystyrene blocks tend to clump together and the polybutadiene blocks tend to clump together. The clusters formed by the polystyrene blocks tie the polybutadiene blocks together. Remember each polybutadiene block has a polystyrene block at each end, and the different polystyrene blocks of the same SBS molecule aren't necessarily in the same cluster. This means that the different polystyrene clusters will be tied together by the polybutadiene blocks.
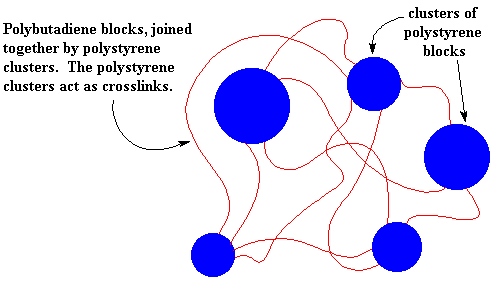
So the polystyrene clusters act as crosslinks for the polybutadiene blocks. And just like the ionic clusters of the ionomers, the polystyrene clusters break up when the SBS is heated, so it can be processed and recycled like a non-crosslinked polymer.
But a thermoplastic elastomer can also be made using a block copolymer made from only one kind of monomer. One can make polypropylene, in which there are blocks of different tacticity, with atactic blocks and isotactic blocks using metallocene catalysis polymerization, as the picture below.
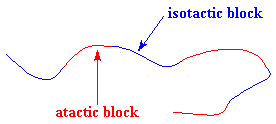
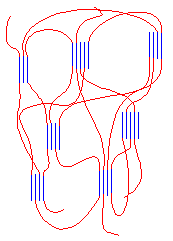
The blocks separate just as they do in SBS rubber. They separate because the isotactic blocks will form crystals, but the atactic blocks are amorphous. The result is something that looks like the following picture. It behaves as an elastomer for the same reasons as SBS rubber does.
This article is reproduced from The Macrogalleria.