Elastomer is a big fancy word, and all it means is "rubber". Some polymers which are elastomers include polyisoprene or natural rubber, polybutadiene, polyisobutylene, and polyurethane. What makes elastomers special is that they can be stretched to many times their original length, and can bounce back into their original shape without permanent deformation.
The reason lies in entropy. Entropy is disorder. Things in our universe like entropy, and tend to become more disordered. The molecules in a piece of rubber, any kind of rubber, have no order to them. They just wind and tangle around each other in one jumbled mess.
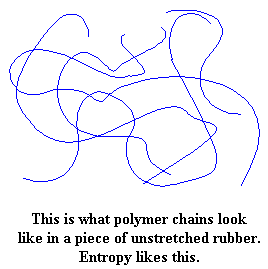
Pull on the piece of rubber, and the molecules are forced to line up in the direction in which the rubber is being pulled. When the molecules line up like this, they become more ordered. If you stretch it far enough, the chains will line up straight enough to crystallize.
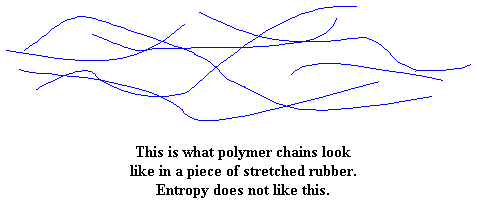
When you let go of this rubber sample you've been stretching, the molecules will quickly go back to their tangled and disordered state. They do this to return to a state of entropy. When this happens, the sample pops back to its original shape.
However, not all amorphous polymers are elastomers. Some are thermoplastics. Whether an amorphous polymer is a thermoplastic or an elastomer depends on its glass transition temperature, or Tg. This is the temperature above which a polymer becomes soft and pliable, and below which it becomes hard and glassy. If an amorphous polymer has a Tg below room temperature, that polymer will be an elastomer, because it is soft and rubbery at room temperature. If an amorphous polymer has a Tg above room temperature, it will be a thermoplastic, because it is hard and glassy at room temperature. So a general rule of thumb is that for amorphous polymers, elastomers have low Tg and thermoplastics have high Tg. But be careful, this only works for amorphous polymers, and not for crystalline polymers.
To help elastomers bounce back even better molecule helps to crosslink them. Crosslinking is the forming of covalent links between the different polymer chains, joining them all into a single networked molecule. Most objects made of rubber contain only one molecule. When the polymer chains are joined together like this, it is even harder to pull them out of their original positions, and so it bounces back even better when stretched. But this makes elastomers hard to recycle. To make recyclable elastomers it needs to tie the molecules together when the rubber is being used, but one which would allow the chains to separate when being processed. The answer is called a thermoplastic elastomer.
This article is reproduced from The Macrogalleria.