When a polymer is made by linking only one type of small molecule or monomer together, it is called a homopolymer. When two different types of monomers are joined in the same polymer chain, the polymer is called a copolymer. For instance, there are two monomers, A and B. They can be made into a copolymer in many different ways.
When the two monomers are arranged in an alternating manner as shown below, the polymer is called an alternating copolymer. One interesting fact about this type is that the ratio of the two monomers is exactly 1:1. However, very few copolymerizations give this type of structure.
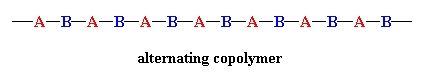
In a random copolymer, the two monomers may follow in any order, as in the figure below. The ratio of the monomers incorporated into the copolymer is a result of a combination of the properties of the monomers, the polymerization conditions and the conversion of the polymerization, to name a few variables. For example, unless the two monomers have exactly the same reactivity, both with the other comonomer and with their own monomers, the ratio in the product will not be exactly 1-to-1. Actually, in most cases it's not, and this leads to a change in the copolymer composition as the reaction proceeds. At the beginning, the more reactive monomer is incorporated more than the less reactive one.
But things change as monomers are used up and the concentration of the more reactive one decreases faster or more than that of the less reactive one. Things even out at some ratio of concentrations, giving polymer that is about 1-to-1 in composition. However, now there's less of the more reactive one, so it's used up faster as the reaction continues, causing the ratio of concentrations to change further until there's mostly just the less reactive monomer present. Copolymers made at this point will have more of the less reactive monomer. While an "average" composition of monomers in the final product might be measured using NMR, FTIR or some other method, the composition of individual chains may (will) be much different than that average. And the total combination of all those copolymer chains, varied in composition as they are, determines the final properties of the material made.
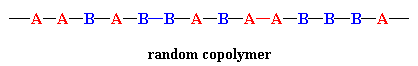
In a block copolymer, all of one type of monomer are incorporated together in one part of the chain, and then all of the other are reacted in somehow. A block copolymer can be thought of as two homopolymers joined together at one of the ends as shown below.
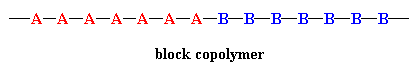
A blocky copolymer that is well known, if you wear shoes, is SBS rubber. It's used for shoe soles and tire treads. "Blocky" means it has some of the characteristics of a true block copolymer but isn't as uniform in composition.
When chains of a polymer made of monomer B are grafted onto a polymer chain of monomer A, a graft copolymer will form as shown below. There are many ways to do this: grafting from, grafting to, or the most controlled way of using a "macromonomer".
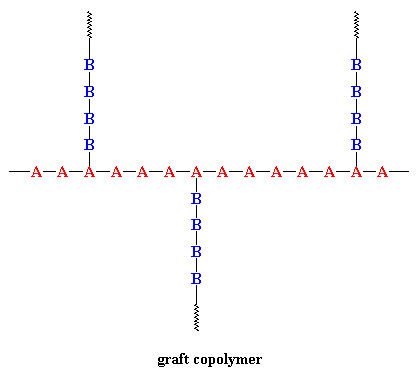
One kind of commercial graft copolymer is high-impact polystyrene (HIPS). It's a polystyrene backbone with chains of polybutadiene grafted onto the backbone. The polystyrene gives the material strength, but the rubbery polybutadiene chains give it resilience to make it tough and less brittle.
This article is reproduced from The Macrogalleria.