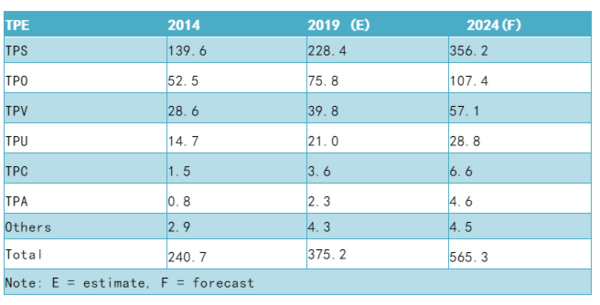
It is estimated that the global market for thermoplastic elastomers (TPEs) for medical and healthcare applications will be of the order of 375,0000 t in 2019. It is likely that this market will grow with a CAGR of 8.5% to 565,000 t by 2024. This represents the highest growth rate of all thermoplastic elastomers. The reason for such growth is a combination of a growing number of the world's population seeking medical care as well as a move to replace a number of incumbent materials, in particular PVC. In addition, certain vulcanised elastomers are also likely to be replaced by thermoplastic elastomers at the same time. The main driving force behind these changes is the need to supply the market with products which have a much higher degree of purity. In Europe in particular, the introduction of the EU medical device and in vitro regulations 2017/745/EU and 2017/746/EU, which will come into force in 2020, will drive the producers of medical devices to examine alternative materials, which fully comply with these new regulations. Other geographical regions will also be forced to comply with these regulations, since from 2020 it will be very difficult to supply the European Union, unless all their products comply as well. TPS can be RF-welded and solvent- bonded, which are two important advantages over polyolefin-based thermoplastic elastomers. It is also now possible to cross¬link SEBS; the main ingredient of TPS. This increases TPS' heat resistance and reduces its compression set. Other TPEs are of course used in the production of medical and health care applications. TPVs also have excellent compression sets and good temperature resistance. They are mainly based on cross-linked EPDM for the moment. TPOs are mainly used in applications such as trays, hospital furniture and other less demanding applications. Tab. : Global market for TPEs by product, 2014, 2019 (E) and 2024 (F) (thousand tonnes) The growth of TPEs in medical and health¬care applications is set for serious growth in the next few years. Despite certain barriers to the use of TPEs, they are slowly but surely taking over from the current incumbents. Their ability to comply with existing and new medical and healthcare regulations is assist¬ing their growth. Doubts about the validity of some plasticisers used in the production of flexible PVC compounds will also allow the continued introduction of TPEs to the growing medical and healthcare markets. Source:TPE Magazine